Abstract
Introduction
The Phase 3 A-LONG study (NCT01181128) and ASPIRE long-term extension (NCT01454739) provide up to 4 years of long-term safety and efficacy data supporting the use of recombinant factor VIII Fc fusion protein (rFVIIIFc) in previously treated adults and adolescents with severe hemophilia A.
Additional longitudinal data are assessed to describe the use of once-weekly dosing with rFVIIIFc. This analysis examined subjects who changed to a once-weekly prophylaxis regimen and reports clinical outcomes with rFVIIIFc from A-LONG through to the third ASPIRE interim data cut (January 11, 2016).
Methods
This analysis evaluated all subjects who participated in A-LONG and the ASPIRE extension study and were ever individualized to a weekly dosing (65 IU/kg every 7 days) interval of rFVIIIFc. A sub-group analysis of subjects who were always on a weekly dosing interval was also conducted.
Annualized bleeding rates (ABR) and adherence rate were evaluated.
Subjects with a target joint (major joint with ≥3 bleeding episodes in a consecutive 6-month period) in the prestudy period were evaluated for target joint resolution (≤2 joint bleeds in a consecutive 12-month period), using International Society on Thrombosis and Haemostasis (ISTH)-defined criteria.
Results
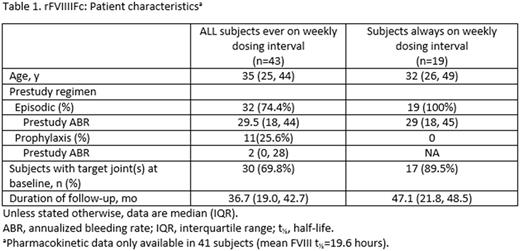
Forty-three subjects were exposed to a weekly dosing interval for a median duration of 36.7 months (3.1 years). Twenty-three subjects started with weekly dosing in A-LONG (19 never switched and 4 switched to more frequent dosing regimens). Fifteen subjects started with more frequent dosing and extended to weekly dosing. Five subjects started with episodic treatment and transitioned to weekly dosing. Thirty-nine of 43 subjects (91%) stayed on weekly dosing once initiated. Patient characteristics and duration of follow-up are listed in the Table. The majority of subjects were on prestudy episodic treatment. Thirty of 43 (69.8%) subjects who were ever on weekly dosing had target joints at baseline. Seventeen of 19 (89.5%) subjects who were always on weekly dosing had target joints at baseline
Median (interquartile range [IQR]) prestudy ABR for subjects on a prestudy episodic regimen was 29.5 (18, 44) in subjects ever on weekly dosing (n=32) and 29 (18, 45) in subjects always on weekly dosing (n=19). On rFVIIIFc, median (IQR) ABR was reduced to 2 (0.7, 5.6) in subjects ever on weekly dosing (n=43) and 1.7 (0.5, 6.7) in subjects always on weekly dosing (n=19). Median spontaneous ABR was 1.2 (0.2, 2.8) in subjects ever on weekly dosing (n=43) and 0.7 (0, 1.6) in subjects always on weekly dosing (n=19). Median (IQR) spontaneous joint ABR was 0.8 (0, 2.5) in subjects ever on weekly dosing (n=43) and 0.2 (0, 1.0) in subjects always on weekly dosing (n=19).
All evaluable target joints in both the ever-on-weekly-dosing groups (n=55) and always-on-weekly-dosing groups (n=29) resolved.
Adherence rates were greater than 90% in both the ever-on-weekly dosing and the always-on-weekly-dosing regimens.
Conclusions
Based on the data from A-LONG/ASPIRE, prestudy ABR and baseline target joint status did not predict a subject's ability to maintain a weekly dosing regimen with rFVIIIFc.
Among subjects who chose to individualize to a weekly dosing regimen on rFVIIIFc, the majority (91%) stayed on weekly dosing and maintained low spontaneous ABR (median=1.2).
There was 100% target joint resolution among subjects who had target joints at baseline.
All subjects were adherent to the weekly dosing regimen for a median of 3.1 years
A weekly dosing regimen may be a reasonable prophylactic option for episodic patients, providing the benefits of prophylactic treatment and reducing treatment burden.
Shapiro: Kedrion Biopharma: Research Funding; Daiichi Sankyo: Research Funding; Selexys: Research Funding; Octapharma: Research Funding; Biogen: Consultancy, Research Funding, Speakers Bureau; ProMetic Life Sciences: Consultancy, Research Funding; PTC Therapeutics: Research Funding; Kedrion Biopharma: Consultancy; Bayer HealthCare: Research Funding; CSL Behring: Research Funding; Baxalta: Consultancy, Research Funding; OPKO: Research Funding; Novo Nordisk: Consultancy. Srivastava: Bayer Healthcare, Shire, Novo Nordisk, Roche Genentech, LFB: Other: Educational grants / Advisory Board / Grants Review / Data Monitoring Committee, Research Funding. Ragni: Biomarin: Consultancy, Honoraria, Research Funding; Alnylam: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria, Research Funding; SPARK: Research Funding; Bayer: Consultancy, Honoraria, Research Funding; MOGAM: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria, Research Funding; NovoNordisk: Honoraria; Sangamo: Research Funding; Genentech/Roche: Research Funding. Pabinger: Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria; Boehringer Ingelheim: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Lethagen: Sobi: Employment, Equity Ownership. Tsao: Bioverativ: Employment. Glazebrook: Bioverativ: Employment. Quon: Baxalta/Shire, Bayer, Bioverativ, CSL, Grifols, Genentech, Novo Nordisk, Pfizer: Consultancy; Baxalta/Shire, Bioverativ, Grifols, Novo Nordisk: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal